Table of Contents
What is crystal structure?
We know that crystal structure deals with the atomic arrangement in the solid crystal structure show the regular three-dimensional pattern of atoms. In geometric terms, crystal structure is known as space lattice or point lattice.
Let’s find out about different types of crystal structure.
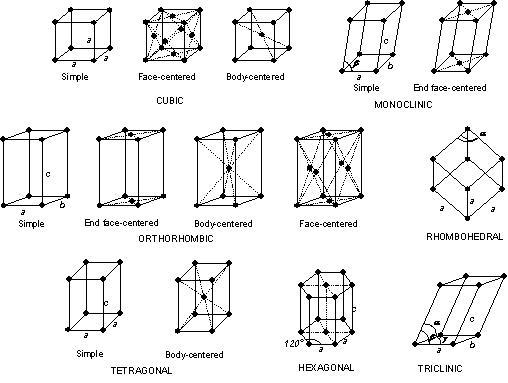
Different types of crystal structures:
There are four crystal structure types as given below:
- Simple Cubic Crystal Structure (SC)
- Body-Centered Crystal Structure (BCC)
- Face Centered Crystal Structure (FCC)
- Hexagonal close packed structure (HCP)
Read About: 5 Best reference books for metallurgy
1. Simple Cubic Crystal Structure (SC) :
In this type of crystal structure, one atom is situated at each corner of the unit cell as shown in the figure. In the simple cubic crystal structure, the total number of atoms is equal to eight.
Simple cubic crystal structure does not have an atom at the center of the unit cell or faces of the unit cell. Now we can say that the average number of atoms per unit in a simple cubic crystal structure one. This type of crystal structure does not exist in any engineering material.
2. Body-Centered Crystal Structure (BCC) :
In body centered crystal structure, one atom is placed at each corner of the unit cell like a simple cubic crystal structure but, in addition to this, there is one atom at the center of the unit cell. A body-centered crystal structure is more complex as compared to the simple cubic crystal structure.
Center atom in the body centered crystal structure does not come in contact with another atom, hence it remains unshared. An average number of atoms per unit cell in body centered crystal structure is two. Metals like Li, K, Na, V, Ta, etc. has this type of crystal structure.
3. Face Centered Crystal Structure (FCC) :
In the face-centered crystal structure, an atom is placed at each corner of the unit cell that is eight corner atoms. One atom is placed at each face center that is six face atoms. In Face centered crystal structure, there is no center atom. In this type of crystal structure, an average number of atoms per unit cell is four. Metals like Cu, Ag, Al, Ca, Pt, etc. contain this type of crystal structure.
Read About: Co-ordination numbers of different crystal structures
4. Hexagonal close packed structure (HCP) :
In Hexagonal close packed structure, crystal structure shows one atom at each corner of the hexagon. Therefore, there are 12 corner atoms in the hexagonal close packed structure. Also, there is one atom on each face of the hexagon.
Interior three atoms in the hexagon remain unshared. In hexagonal close packed structure, an average number of atoms per unit cell is six. Metal like Zn, Co, Cd, Mg, Be, Ca, etc. has this type of crystal structure.
Conclusion:
We have discussed in detail about crystal structure and crystal structure types such as simple cubic, body centered, face centered, and hexagonal close packed crystal structure. If you have any queries about this article then feel free to share it in the comment box.
You’re doing a great job Man, Keep it up.
What is the the tetrahedral structure?